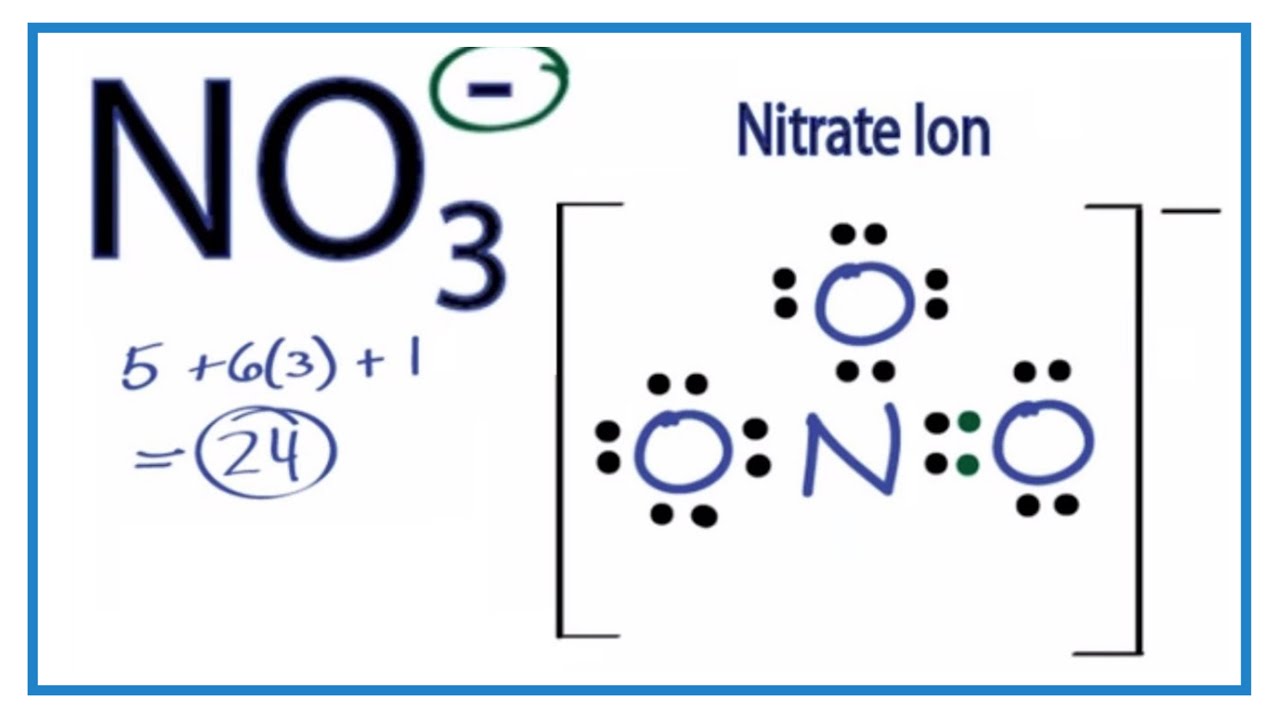
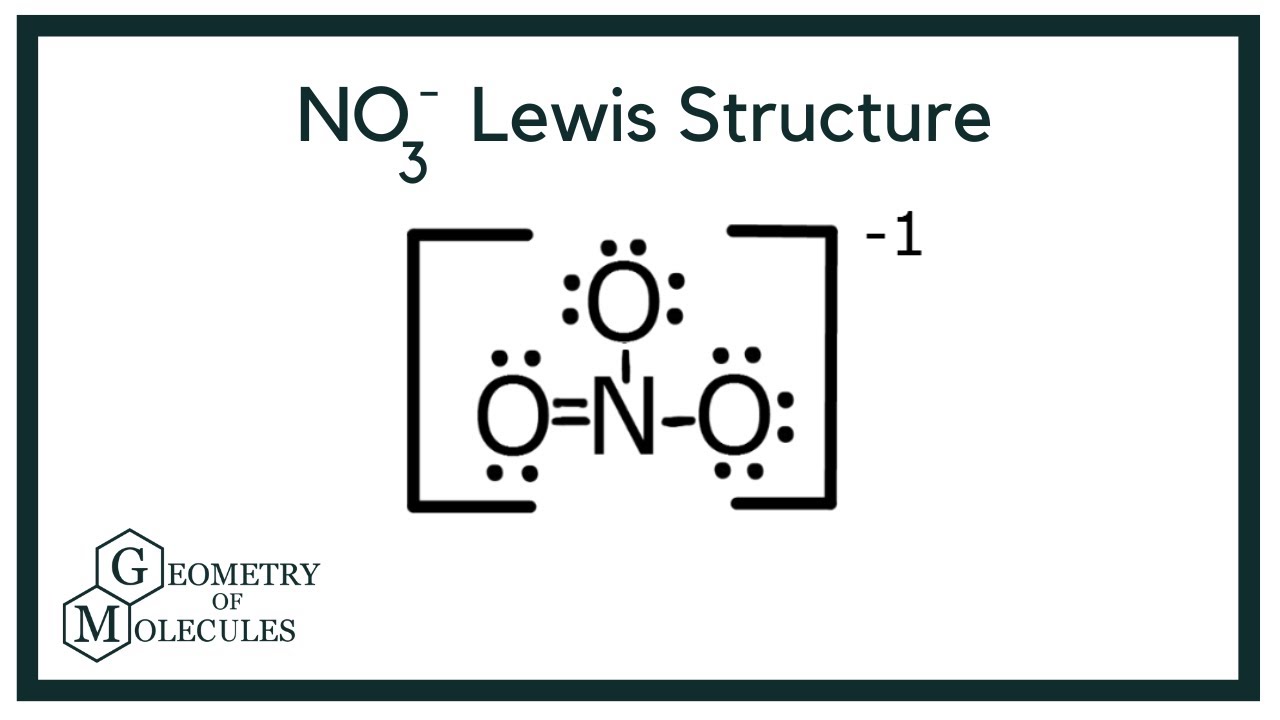
Understanding The Lewis Structure Of NO3-
The structure of the nitrate ion (NO3-) depicts the arrangement of atoms and electrons within the ion. This representation utilizes Lewis dot symbols to show valence electrons, bonding pairs, and lone pairs of electrons. The structure reveals how the nitrogen atom is bonded to three oxygen atoms, and how the negative charge is distributed among the atoms. A central nitrogen atom is typically shown double-bonded to two oxygen atoms and single-bonded to one. All oxygen atoms have a negative charge based on the octet rule.
Understanding this structure is fundamental to predicting the reactivity and properties of the nitrate ion. It provides a visual model for comprehending its behavior in chemical reactions, particularly its role as an oxidizing agent. The resonance structures involved highlight the delocalization of the negative charge, which stabilizes the ion and affects its interactions with other substances. This knowledge is critical for diverse applications, including understanding the behavior of fertilizers, explosives, and various biological systems.
Further exploration of the nitrate ion will delve into its reactions and its applications, drawing connections to various scientific disciplines, including chemistry, biochemistry, and environmental science. This knowledge is essential for understanding the role of the nitrate ion in various ecological processes and human endeavors.
What is the Lewis Structure for NO3-?
The Lewis structure for the nitrate ion (NO3-) visually represents the arrangement of atoms and electrons. Understanding this structure provides insight into the ion's properties and behavior.
- Central Atom: Nitrogen
- Multiple Bonds: Double
- Oxygen Atoms: Three
- Formal Charges: Resonance
- Electron Placement: Octet Rule
- Structure Shape: Trigonal planar
The nitrate ion's structure reveals nitrogen bonded to three oxygen atoms. Crucially, resonance structures demonstrate delocalization of the negative charge, stabilizing the ion. This delocalization impacts the ion's reactivity and its role as an oxidizing agent, which is crucial in various chemical processes, including in fertilizers and explosives. The trigonal planar shape arises from the arrangement of bonds and lone pairs around the central nitrogen, a feature essential for understanding its interactions in solutions and reactions. The structure emphasizes the dynamic and shared nature of electrons in molecules, offering a fundamental foundation for studying molecular interactions in a variety of chemical contexts.
1. Central Atom
The central atom in the nitrate ion (NO3-) dictates the fundamental structure and properties of this key chemical species. Understanding the role of nitrogen as the central atom is crucial for comprehending the Lewis structure, as it directly influences the bonding arrangements and charge distribution within the ion. This foundational aspect is essential for predicting reactivity, stability, and various chemical behaviors of the nitrate ion.
- Bonding Arrangements:
Nitrogen, positioned centrally, forms bonds with the three oxygen atoms. The nature of these bondssingle, double, or a combinationand their distribution are directly tied to the overall structure. Different bonding configurations, though contributing to a valid Lewis structure, lead to distinct molecular geometries and charge distributions. This aspect underscores how the central atom's role shapes the fundamental structure of the ion. Delocalization of electrons, a characteristic consequence of specific bonding arrangements, is also fundamentally impacted by the position of the nitrogen atom.
- Charge Distribution:
The central nitrogen atom's electronegativity, relative to oxygen, plays a significant role in how the negative charge, inherent to the nitrate ion, is distributed. The arrangement of bonds and electron pairs around nitrogen dictates the formal charges on each atom and the overall charge distribution within the molecule. The distribution of charge impacts the ion's behavior in chemical reactions and its interactions with other species.
- Molecular Geometry:
The location of the nitrogen atom directly affects the overall molecular geometry, particularly the angle between the bonds to the surrounding oxygen atoms. The resulting shape affects how the nitrate ion interacts with other molecules or ions. Delocalization, driven by the central nitrogen atom, leads to a trigonal planar shape which in turn affects the ion's polarity and reactivity.
In summary, the nitrogen atom's central position in the nitrate ion's structure is paramount to understanding its Lewis representation. The bonding arrangements, charge distribution, and molecular geometry, all stemming from this central position, significantly affect the nitrate ion's chemical characteristics and its role in various chemical and biological processes. The fundamental importance of this central atom in shaping the structural and functional properties cannot be overstated.
2. Multiple Bonds
Double bonds in the Lewis structure for the nitrate ion (NO3-) are integral to representing the bonding arrangement and electron distribution. Their presence significantly impacts the ion's stability and reactivity, features critical for understanding the nitrate ion's role in various chemical processes.
- Bonding Configuration and Electron Delocalization
The presence of double bonds in NO3-, specifically the nitrogen-oxygen double bonds, is not as straightforward as a single pair of shared electrons. Instead, the structure exhibits resonance, with the electrons being delocalized over multiple oxygen atoms. This delocalization, a consequence of the double bonds, leads to significant stabilization of the nitrate ion, making it a more stable species compared to structures with only single bonds. This phenomenon of delocalization is a core concept in understanding the behavior of molecules like NO3-. The existence of multiple bonding arrangements (resonance) affects the nature of the chemical bonds.
- Formal Charge and Stability
The presence of double bonds and the resulting resonance in NO3- directly influence the formal charges on the atoms. This, in turn, significantly impacts the overall stability of the ion. The delocalization of electrons through multiple bonds reduces the formal charge on each atom, enhancing stability and allowing for a more balanced electron distribution. The formal charge distribution is a crucial determinant in the overall structural stability of the nitrate ion.
- Effect on Molecular Geometry
The presence of double bonds, alongside single bonds and lone pairs, dictates the overall geometry of the nitrate ion. The delocalized electrons from the double bonds, along with the other electron pairs, create a trigonal planar molecular geometry for the nitrate ion. The geometry is crucial for understanding how the nitrate ion interacts with other molecules, significantly impacting its reactivity and overall behavior. The precise arrangement of atoms influences the molecule's polarity and its potential to form various types of chemical bonds.
- Influence on Reactivity and Properties
The specific bonding arrangements, including the presence of double bonds and the resulting resonance structures, strongly influence the nitrate ion's reactivity. This is directly tied to the ion's ability to act as an oxidizing agent, a characteristic crucial in chemical processes ranging from industrial applications to biological systems. The stability conferred by multiple bonds, combined with the delocalized electrons, makes the nitrate ion a potent oxidizing agent, further influencing its potential reactions.
In conclusion, the double bonds in the Lewis structure of nitrate underscore the significance of resonance, delocalization, and stability in understanding chemical compounds like nitrate. The combination of factors related to bonding configurations, formal charge, and molecular geometry determines the crucial properties that shape the nitrate ion's role in various chemical contexts.
3. Oxygen Atoms
The presence of three oxygen atoms in the nitrate ion (NO3-) is not merely a numerical detail; it's a crucial component defining the ion's structure, stability, and reactivity. The arrangement of these oxygen atoms around the central nitrogen atom directly influences the bonding patterns and charge distribution, ultimately shaping the molecule's overall properties. This fundamental aspect underpins the ion's function in various chemical processes.
The three oxygen atoms necessitate the formation of multiple bonding configurations, specifically double and single bonds, to satisfy the octet rule for each atom. This leads to a phenomenon called resonance, where multiple valid Lewis structures contribute to the true structure of the ion. This delocalization of electrons over the oxygen atoms significantly stabilizes the nitrate ion, reducing the formal charges and enhancing its resistance to decomposition. The need to accommodate three oxygen atoms, coupled with the requirement of minimizing formal charge, dictates the presence of delocalized pi electrons, a characteristic that further determines the molecule's stability and reactivity. Real-world examples include the use of nitrates in fertilizers, explosives, and various industrial processes. The stability and reactivity of nitrate directly stem from its specific molecular arrangement, influenced by its oxygen atoms and nitrogen central atom.
In summary, the presence of three oxygen atoms in the nitrate ion is fundamental to its structure and properties. This arrangement, coupled with the necessity of the octet rule and the resulting resonance structures, dictates the stability, bonding patterns, and eventual reactivity of the nitrate ion. Comprehending this foundational aspect is critical for understanding the nitrate ion's role in various chemical processes, from its use as a crucial component in fertilizers to its presence in certain types of explosives. The careful consideration of the specific molecular arrangement involving three oxygen atoms is essential in applying accurate predictions for chemical reactions and behaviors involving nitrate.
4. Formal Charges
The concept of resonance, crucial to understanding the Lewis structure of the nitrate ion (NO3-), arises from the need to represent the delocalization of electrons. Formal charges play a critical role in illustrating this phenomenon. The nitrate ion, with its three equivalent oxygen atoms bonded to a central nitrogen, necessitates multiple Lewis structures to adequately depict electron distribution. Each resonance structure differs in the placement of double bonds between nitrogen and oxygen atoms, resulting in distinct formal charges on the atoms. Without considering resonance, a single Lewis structure would assign uneven formal charges, potentially misrepresenting the ion's actual stability and behavior.
Resonance structures are not independent entities; instead, they contribute to a single, composite structure. This composite structure, where electrons are delocalized over multiple atoms, is more stable than any single contributing structure. The concept of resonance, in tandem with the formal charges inherent in each resonance contributor, directly elucidates the delocalization of electrons and, ultimately, the stability of the nitrate ion. This stability, reflected in the evenly distributed negative charge, is a key factor in the nitrate ion's role as an oxidizing agent in numerous chemical reactions, including those occurring in fertilizers and explosives. The practical significance lies in accurately predicting the ion's reactivity, which is essential for safe handling and applications of nitrate compounds.
In essence, formal charges, in conjunction with resonance structures, are essential tools for accurately representing the true electron distribution within the nitrate ion. The delocalization represented by resonance significantly enhances the ion's stability, affecting its chemical behavior and making it a critical component in various chemical and industrial processes. Understanding these concepts is pivotal for predicting reactivity, ensuring safety, and optimizing the utilization of nitrate compounds in diverse applications.
5. Electron Placement
The octet rule is a fundamental principle in chemistry governing the arrangement of electrons in atoms, particularly relevant when visualizing molecular structures like the nitrate ion (NO3-). Understanding electron placement according to the octet rule is crucial for constructing accurate Lewis structures, which depict bonding and electron distribution within molecules. The rule's application dictates the arrangement of atoms and electrons within the nitrate ion, ultimately affecting its chemical behavior and properties.
- Nitrogen's Role:
The central nitrogen atom in the nitrate ion strives to achieve a stable electron configuration. This involves forming bonds with oxygen atoms to acquire eight valence electrons, satisfying the octet rule. The way nitrogen bonds influences the overall structure and charge distribution within the nitrate ion. The fulfillment of the octet rule for nitrogen, as well as for the surrounding oxygen atoms, shapes the molecule's overall stability.
- Oxygen's Contribution:
Each oxygen atom in the nitrate ion aims to achieve a full octet. Oxygen atoms achieve this through bonding with nitrogen and, in some cases, through the presence of lone pairs of electrons. This need for a complete octet influences the number and types of bonds that form, impacting the molecular structure and the final arrangement of electrons. The contribution of each oxygen atom to the overall electron configuration is critical in understanding the stability and reactivity of the entire nitrate ion.
- Resonance and the Octet Rule:
The octet rule, while a guiding principle, sometimes requires multiple resonance structures to accurately reflect the observed stability of the nitrate ion. Each resonance structure fulfills the octet rule for the nitrogen and each oxygen, but the actual structure is a composite of these contributors. The octet rule, coupled with the principle of resonance, provides a dynamic model for electron placement and molecular structure, highlighting the ability of electron systems to achieve a stable configuration in multiple valid arrangements. The true structure exists as a hybrid of all these possible resonance forms.
- Negative Charge and Electron Placement:
The presence of a negative charge on the nitrate ion modifies the electron placement slightly. The extra electron, necessary to account for the negative charge, affects the distribution of electrons around the nitrogen and oxygen atoms to satisfy the octet rule while maintaining the overall negative charge. The placement of this additional electron is important in understanding the electrostatic interactions and the chemical behavior of the nitrate ion.
In conclusion, the octet rule acts as a critical guideline for establishing the Lewis structure of the nitrate ion. The need for each atom to achieve an octet, along with resonance and the effect of the negative charge, dictates the arrangement of atoms and electrons, ultimately influencing the stability and behavior of the nitrate ion in chemical reactions. The interaction of these principles creates a comprehensive model for understanding the structure and reactivity of nitrate.
6. Structure Shape
The trigonal planar shape of the nitrate ion (NO3-) arises directly from the arrangement of atoms and electrons within its Lewis structure. The central nitrogen atom, bonded to three oxygen atoms, and the delocalized nature of electrons, as reflected in resonance structures, dictate the specific geometry. The three oxygen atoms, positioned around the nitrogen atom, and the presence of double bonds (and their resonance) create an equilateral triangular arrangement in the plane. The concept of delocalized electrons, integral to the resonance structures, results in this flat, planar shape, rather than a three-dimensional structure, with bond angles of approximately 120 degrees.
This trigonal planar geometry significantly impacts the nitrate ion's properties and reactivity. The flat structure facilitates interactions with other molecules or ions, influencing its role as an oxidizing agent. The symmetrical distribution of atoms in a plane minimizes electron repulsion, contributing to the ion's stability. In practical applications, the planar nature of nitrate is crucial for understanding its behavior in various chemical processes, including the manufacturing of fertilizers (where the orientation of the ion within the crystal lattice is critical), and in explosive formulations. The planar geometry influences the way the nitrate ion bonds with other elements in these applications.
In summary, the trigonal planar shape of the nitrate ion is a direct consequence of its Lewis structure, particularly the arrangement of atoms and the delocalization of electrons. This planar geometry is not merely a geometric detail but is essential for understanding and predicting nitrate's behavior in chemical reactions and applications. The predictable interaction with other species, resulting from the trigonal planar geometry, makes the nitrate ion a valuable and sometimes hazardous component in various chemical systems. The ion's symmetry and planar geometry underpin its crucial roles in different industrial and biological settings, making the relationship between its structure and shape a pivotal element in chemistry.
Frequently Asked Questions about the Lewis Structure of NO3-
This section addresses common inquiries regarding the Lewis structure of the nitrate ion (NO3-), providing concise and informative answers to facilitate a deeper understanding of this crucial chemical species.
Question 1: What is a Lewis structure, and why is it important for NO3-?
A Lewis structure visually represents the arrangement of atoms and electrons in a molecule or ion. For NO3-, it shows the bonding and non-bonding electron pairs, highlighting the distribution of valence electrons. This representation is important for understanding the bonding nature, stability, and ultimately, the reactivity of NO3-. Accurate depiction of the electron configuration is fundamental to predicting how the ion interacts with other species and facilitates accurate modeling of its behavior in chemical reactions.
Question 2: How are multiple bonds represented in the Lewis structure of NO3-, and what does this imply?
Multiple bonds, specifically double bonds, are shown in the Lewis structure of NO3-, but crucially, not in a fixed way. The structure employs resonance, depicting multiple valid arrangements of double and single bonds around the nitrogen atom. This resonance implies that the true structure is a hybrid of all contributing forms, showcasing delocalized electrons. The delocalization significantly enhances the stability of the nitrate ion, making it a more robust chemical entity.
Question 3: Why are there three oxygen atoms in the nitrate ion, and how does this affect the structure?
The presence of three oxygen atoms is a consequence of the need for a stable electron configuration, specifically satisfying the octet rule for each atom, while also minimizing formal charges. This arrangement necessitates multiple bonding configurations and resonance, resulting in a trigonal planar structure where all atoms reside in a plane. The three oxygen atoms lead to a unique electron distribution pattern, impacting the ion's stability and reactivity.
Question 4: What are formal charges in the context of nitrate, and why are they relevant?
Formal charges are hypothetical charges on atoms assuming equal sharing of electrons in bonds. Calculating formal charges in NO3- helps evaluate the most plausible resonance structure by minimizing charges. The resonance structures contribute to a more stable, delocalized electron system, where the negative charge isn't confined to a single atom but distributed across the entire ion.
Question 5: How does the octet rule relate to the Lewis structure of NO3-?
The octet rule, which states that most atoms strive to attain eight electrons in their valence shell, guides the arrangement of atoms and electrons in the Lewis structure. Nitrogen and each oxygen atom fulfill this rule, either through direct bonds or lone pairs. The rule's influence, particularly on minimizing formal charges and enabling resonance, highlights its importance in predicting the arrangement within the nitrate ion.
Question 6: What is the significance of the trigonal planar shape for NO3-?
The trigonal planar shape is a direct consequence of the distribution of electrons and bonds around the central nitrogen atom. This planar geometry affects the overall stability and reactivity of the nitrate ion. It allows for specific interactions with other molecules, reflecting the influence of its shape on its chemical behavior.
In summary, the Lewis structure of NO3-, including its resonance structures, formal charges, and the influence of the octet rule, is critical for understanding the stability and reactivity of the nitrate ion. This knowledge is fundamental to predicting its behavior in diverse chemical environments.
The following section will delve into the applications and importance of nitrate ions in various contexts.
Tips for Determining the Lewis Structure of NO3-
Accurate representation of molecular structures, such as the nitrate ion (NO3-), is crucial for understanding chemical behavior. These tips offer a structured approach to constructing the correct Lewis structure, focusing on key principles.
Tip 1: Identify the Central Atom. In NO3-, nitrogen (N) is the least electronegative atom, typically occupying the central position. Understanding this fundamental placement is the first step in correctly constructing the Lewis structure.
Tip 2: Count Valence Electrons. Nitrogen has five valence electrons, and each oxygen has six. The negative charge on the nitrate ion adds one more electron. Summing these yields a total of 24 valence electrons.
Tip 3: Draw Single Bonds to Connect Atoms. Begin by connecting the three oxygen atoms to the central nitrogen atom with single bonds. This accounts for six bonding electrons.
Tip 4: Satisfy the Octet Rule for Oxygen Atoms. Each oxygen atom needs eight electrons in its outermost shell. Complete the octets of the oxygen atoms by adding lone pairs. This step accounts for 18 more electrons (9 pairs).
Tip 5: Determine Remaining Electrons and the Central Atom. With 18 bonding electrons accounted for, six electrons remain. These are positioned as a double bond between one oxygen atom and the nitrogen atom, completing nitrogen's octet. The remaining oxygen atoms require a single bond each.
Tip 6: Assess Formal Charges. Formal charges help assess the most likely structure. Calculate the formal charge on each atom (using the formula: valence electrons - (lone pair electrons + bonding electrons/2)). Ideally, all formal charges should be minimized, or zero if possible. Resonance will occur when multiple valid structures have similar charge distributions.
Tip 7: Apply Resonance Structures. Because of resonance, a single structure does not completely represent the true distribution of electrons. Draw alternative structures that vary the locations of double bonds between nitrogen and oxygen while still obeying the octet rule. The actual structure is a composite of these contributing resonance structures.
By following these detailed steps, a complete and accurate representation of the Lewis structure of the nitrate ion, emphasizing resonance, can be generated.
These tips provide a comprehensive approach to visualizing the electron distribution within the nitrate ion, enabling a deeper understanding of its behavior in chemical reactions.
Conclusion
The Lewis structure of the nitrate ion (NO3-) serves as a fundamental representation of its electronic configuration and bonding arrangements. This structure reveals a crucial aspect of the ion's properties: resonance. The delocalization of electrons, depicted through multiple resonance structures, significantly contributes to the ion's stability. The interplay of single and double bonds between nitrogen and oxygen, coupled with the crucial role of the central nitrogen atom, dictates the trigonal planar geometry and the consistent negative charge distribution throughout the ion. The octet rule, governing electron distribution, plays a critical role in determining the arrangement of atoms and electrons within the nitrate ion. This structural representation underpins the ion's role as an oxidizing agent in chemical reactions, a characteristic with practical implications in various contexts, from fertilizer production to explosive formulations. The ability to accurately depict the structure empowers predictive analyses of chemical behavior and facilitates understanding of the ion's role in diverse chemical systems.
Further investigation into the nitrate ion's reactivity and applications is crucial. A deeper understanding of its interactions within various chemical and biological systems can lead to advancements in areas such as sustainable agriculture, materials science, and environmental remediation. Continued study of this fundamental chemical species will undoubtedly reveal further insights into the intricate relationship between molecular structure and chemical function.
Article Recommendations
- Expecting A Baby Ashantis Pregnancy Journey
- Sylvester Stallones 80s Iconic Action Rocky Returns
- Top Muscular Actresses Fierce Fit Female Stars